Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated. Calculate the degree of unsaturation for each of the following molecular formulas.
 Degree Of Unsaturation Chemistry Libretexts
Degree Of Unsaturation Chemistry Libretexts
Alternatively to calculate the number of hydrogen atoms enter values for the degree of unsaturation a c d and e and press calculate.
Calculate degree of unsaturation. General formula The formula for degree of unsaturation is. Hence the DoB formula divides by 2. An alkyne has two degrees of unsaturation 2 pi bonds and an aromatic ring has four 3 pi bonds plus a ring.
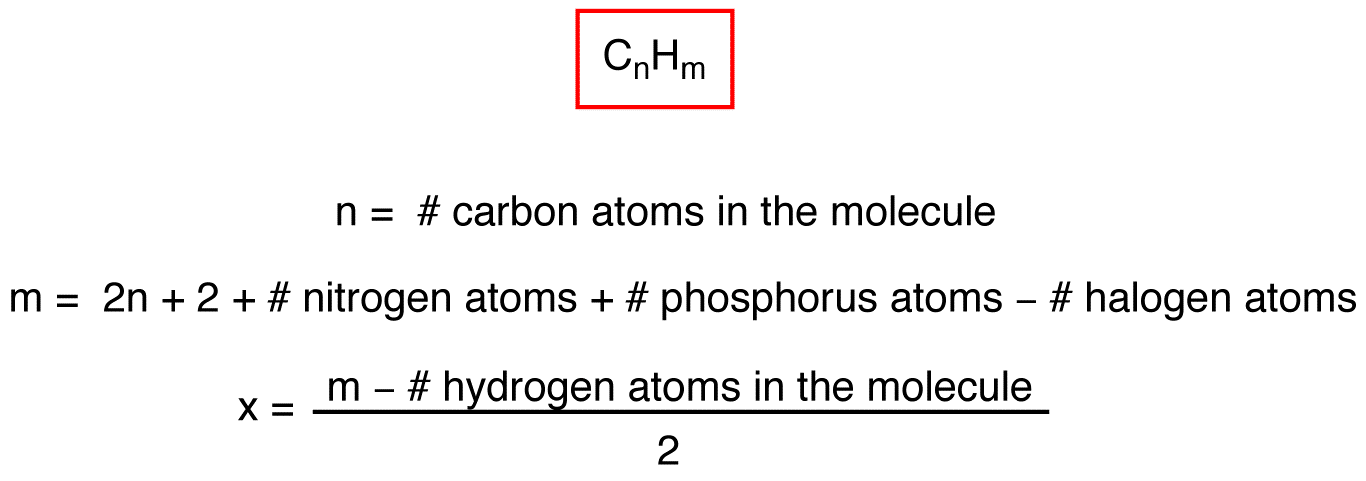
Where ni is the number of atoms with valence vi. A degree of unsaturation of 0 means that the molecule follows the formula for an acyclic alkane C n H 2n 2. The degree of unsaturation tells you how may rings and multiple bonds are present in a compound provided you know the molecular formula of the compound.
General formula of the degree of unsaturation To calculate the unsaturation formula firstly we have to know the molecular formula of the molecule that has the form of C_ V H_ W N_ X O_ Y X_ Z C V. Calculating Degrees of Unsaturation DoUIf the molecular formula is given plug in the numbers into this formula. A C 6 H 10 b C 5 H 10 O 2 c C 5 H 9 N d C 3 H 5 ClO e C 10 H 20 f C 4 H 6 Br 2 g C 6 H 6 h C2Cl6 i C 2 H 4 O 2 j C 10 0H 200 C l2 O 16 View Answer.
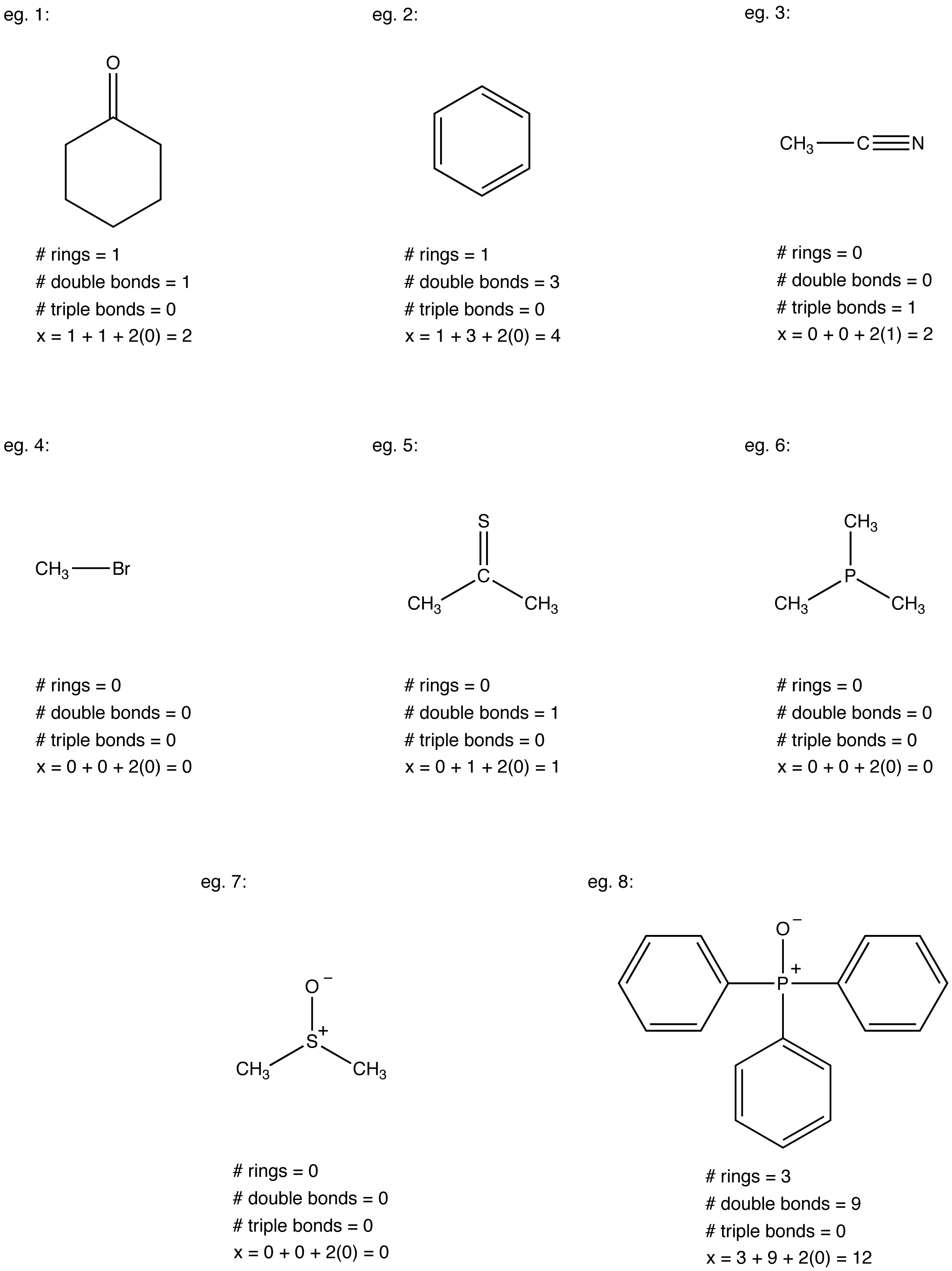
Each degree of unsaturation refers to a decrease in two hydrogens in the molecule as a result of the presence of a pi bond or a ring. Enter the molecular formula in the input field Step 2. Knowing the degrees of unsaturation make it easier to figure out the molecular structure and also helps when double-checking the number of pi bonds andor cyclic rings.
Calculate the degree of unsaturation aka index of hydrogen deficiency to limit the number of possible structures. Calculating The Degree of Unsaturation DoU C is the number of carbons N is the number of nitrogens X is the number of halogens F Cl Br I H is the number of hydrogens. Calculate the degree of unsaturation for each of the following molecular formulas.
Remember each degree of unsaturation is a ring or pi bond likely an alkene or carbonyl. DoU 05 x 2C 2 N - X - H 05 x 12 2 0 - 1 - 11 1 and the unknown therefore contains either a. Go to your Tickets dashboard to see if you won.
The molecule contains one degree of unsaturation from the molecular formula since. That is an atom that has a valence of x contributes a total of x 2 to the degree of unsaturation. Plugging this reduced formula into the preceding equation gives five degrees of unsaturation for the molecular formula C 8 H 6 F 3 NO 2.
Degree of unsaturation 12 2 2a - b c - e To calculate the degree of unsaturation enter the values of a b c d and e and press calculate. AC6H8 bC40H56 cC10H16O2 dC8H9Br eC8H9ClO fC7H11N gC4H8BrN. MathrmC_mathrmg mathrmH_1 mathrmO_2 The Study-to-Win Winning Ticket number has been announced.
Degrees of unsaturation can help us determine how an alkane molecule will act. In other words both the formula C 8 H 6 F 3 NO 2 and the formula C 8 H 8 have identical numbers of degrees of unsaturation. The formula subtracts the number of Xs because a halogen X replaces a hydrogen in a compound.
This gives a reduced equation of C 8 H 631 C 8 H 8. This organic chemistry video tutorial explains how to calculate the degree of unsaturation or the index of hydrogen deficiency of a molecule given its molecu. Now click the button Calculate Degree of Unsaturation to get the result Step 3.
Solution for Calculate the number of degrees of unsaturation for each molecular formula. This organic chemistry video tutorial provides the equation needed to calculate the degree of unsaturation also known as the index of hydrogen deficiency fro. Finally the degree of unsaturation for the given molecular formula will be displayed in the output field.
DoU2C2-NX-H2 C is the number of carbons N is the number of nitrogens X is the number of halogens F Cl Br I H is the number. Calculating Degrees of Unsaturation DoU C is the number of carbons N is the number of nitrogens X is the number of halogens F Cl Br I H is the number of hydrogens.