Ionic compounds - AQA. Suppose that you want to name the compound that results from the reaction of lithium and sulfur.
 How To Name Ternary Ionic Compounds
How To Name Ternary Ionic Compounds
The second part is the name of the nonmetal element with the suffix -ide.

How to name ionic compounds. In this tutorial we will be learning how to name ions and ionic compounds from the formula and how to find the formula from the compound name. The cation has the same name as its element. Jot down the formula of the ionic compound.
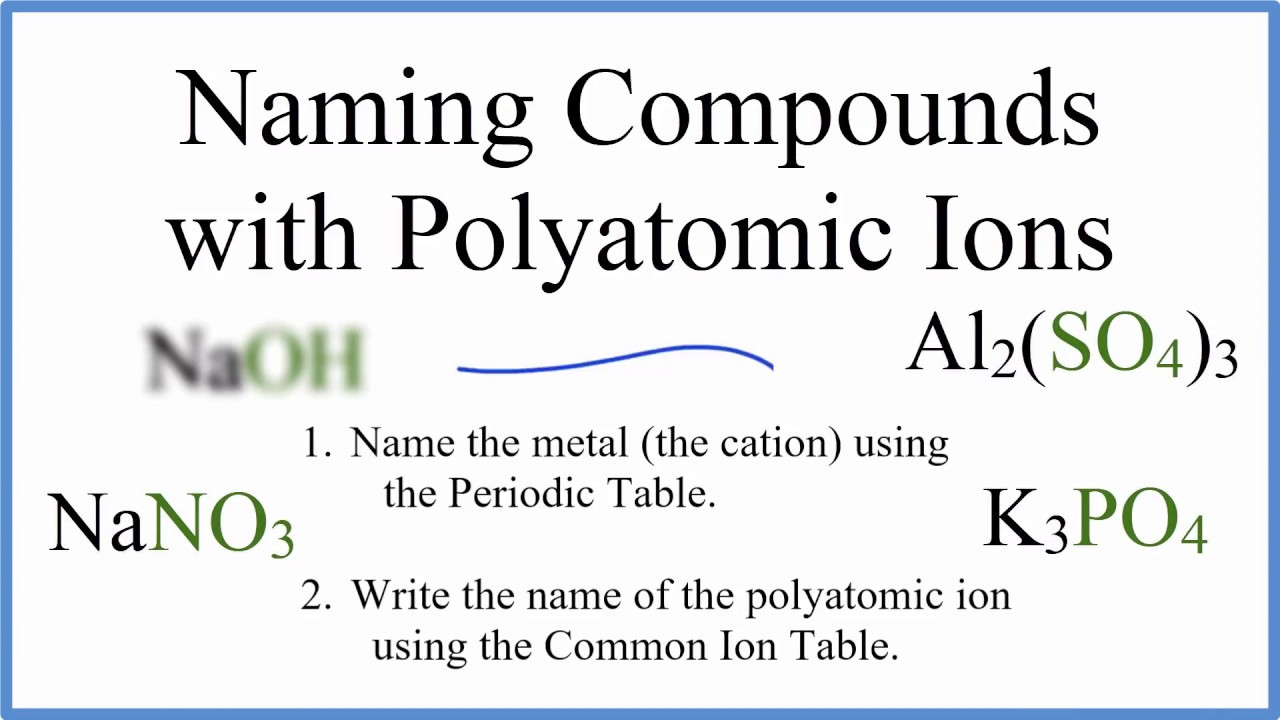
An ionic compound is named first by its cation and then by its anion. When an ion consists of more than one element we refer to it as a polyatomic ion To recognize an ionic compound look for the presence of a metal or a known polyatomic ion - once you find one you more than likely have an ionic compound. An ionic compound is made up of charged particles called ions.
Naming Ionic Compounds Using -ous and -ic. When you name binary ionic compounds with transition metals the rules are the same as those of binary ionic compounds. The cation has the same name as its element.
It has a giant lattice structure with strong electrostatic forces of attraction. Ions of either variety may contain either a single element or more than one element. Left of the metalloid staircase it is a metal and the compound is ionic When naming ionic compounds we never indicate the ratio of component elements in the name - that is for covalent compounds only.
If the first element you see in the compound is found on the left side of the periodic table ie. The names for transition metal compounds often have roman numerals in them beca. Some examples of names for ionic compounds.
Consult the periodic table of elements. The first part of the name is the name of the metal element. You first write the name of the metal lithium and then write the name of the nonmetal adding an -ide ending so that sulfur becomes sulfide.
The Roman numeral naming convention has wider appeal because many ions have more than two valences. Well learn how to name ionic compounds that have transition metals in them. Ionic and molecular compounds are named using somewhat-different methods.
For a two element ionic compound the naming is simple. The anion is named by taking the elemental name removing the ending and adding -ide. NaCl Sodium Chloride.
The ionic lattice An ionic compound is a giant structure of ions. These endings are added to the Latin name of the element eg stannousstannic for tin to represent the ions with lesser or greater charge respectively. Lets say the ionic compound youre working with is NaCl.
When you name ionic compounds you write the name of the metal first and then the nonmetal. Naming Basic Ionic Compounds. Use a pen or.
The ions have a regular repeating arrangement called an ionic lattice. When naming ionic compounds all of the information youll need is on the periodic table. The anion is named by taking the elemental name removing the ending and adding ide.
Although Roman numerals are used to denote the ionic charge of cations it is still common to see and use the endings -ous or -ic. This lesson will demonstrate how to write a chemical formula for a binary ionic compound when given the systemic name. With my strategy and step by step examples you will be naming compounds like a pro in no time.
It has a giant lattice structure with strong electrostatic forces of attraction. Google Classroom Facebook Twitter. Ionic compounds - AQA.
For example K 1 is called the potassium ion just as K is called the potassium atom. For example K 1 is called the potassium ion just as K is called the potassium atom. For binary ionic compounds ionic compounds that contain only two types of elements the compounds are named by writing the name of the cation first followed by the name of the anion.
An ionic compound is made up of charged particles called ions. The name of the metal is written first followed by the name of the nonmetal with its ending changed to ide. For example KCl an ionic compound that contains K and Cl- ions is named potassium chloride.
For example K 2 O is called potassium oxide. Naming compounds have never been so simple. Binary ionic compounds typically consist of a metal and a nonmetal.
An ionic compound is named first by its cation and then by its anion. The lattice is formed because the ions attract each other.
How to write an ionic equation. Identify the states of matter of each compound in the equation.
How To Write And Balance A Net Ionic Equation
Balance the chemical equation.

How to write a net ionic equation. In one of them electrons are being lost an oxidation process and in the other one those electrons are being gained a reduction process. A net ionic equation is derived from a balanced chemical equation so we need to. Fill in the state symbols You need to be familiar with the solubility table to get this part right Split all aqueous substances Exception of halogens during displacement Cancel spectator ions ions that remain unchanged on the LHS and RHS of the chemical equation Viola.
Determine the physical states of all reactants and products. Balance the chemical equation. Before writing a net ionic equation you must first make sure your starting.
Ionic Equations for Neutralisation. Write and balance the molecular equation first making sure that all formulas are correct. Any redox reaction is made up of two half-reactions.
Balance the complete molecular equation. In the complete ionic equation soluble ionic compounds and strong acids are rewritten as dissociated ions. In an ionic equation.
Oftentimes you will be able to identify keywords in. Then write the ionic equation showing all aqueous substances as ions. Break up all the aq compounds into its ions this is TOTAL3.
Write a balanced net ionic equation for this reaction. How to write net ionic equations for redox reactions. Start text C l end text start superscript minus end superscript.
Writing a Net Ionic Equation 1. In the net ionic equation any ions that do not participate in the reaction called spectator ions are excluded. For an insoluble combination write the formula for that compound as a product solid state.
Writing ionic equation is extremely similar to writing chemical equations. Spectator ions are ions that remain. If all combinations are soluble there is no reaction and thus no net ionic equation to write.
Determine what species. It explains how to predict the products of double replacement reactions and acid ba. If you dont have a net ionic equation to balance that means essay marking rubric you have what is usually called a molecular equation or a complete molecular equation.
Half-reaction method depends on the division of the redox reactions into oxidation half and reduction half. Write and balance the molecular equation first making sure that all formulas are correct. Finally eliminate spectator ions and write the net ionic equation.
Recall that ionic compounds that dissolved in water will dissociate completely into ions have charge. Now we must identify the physical state of each. This chemistry video tutorial explains how to balance and predict the products of precipitation reaction in addition to writing the net ionic equation.
Lets demonstrate this by writing the ionic equation for the neutralisation of hydrochloric acid with sodium hydroxide. Carry through any coefficients. Write a balanced chemical equation2.
Cancel out spectator ions. This video covers how to predict products how to balance a chemical equation how to identify the solubility of a compound how to write a complete ionic e. How to write total and net ionic equations1.
How To Write An Ionic Equation In 4 Simple Steps. Any aq that. Write the equation in terms of all of the ions in the solution.
These two equations are described as electron-half-equations or half-equations or ionic-half-equations or half-reactions - lots of variations all meaning exactly the same thing. The other ions remain in solution and should also be written on the product side but as aqueous ions. Therefore the net ionic equation will show the actual chemical change without the spectator ions.
By convention a net ionic equation is written with the smallest possible integer values for the stoichiometric coefficients. This chemistry video tutorial explains how to write net ionic equations. Number of atoms of each elements must be balanced.
As a result the net ionic equation shows only the species that are actually involved in the chemical reaction. Write a balanced chemical equation for the reaction. Write the equation and balance it if necessaryNaCl aq AgNO 3 aq AgCl s NaNO 3 aq Step 2.
There are three steps to writing a net ionic equation. Then write the ionic equation showing all aqueous substances as ions. In the net ionic equation all species with s l and g will be unchanged.
In other words break all of the strong electrolytes. Only compounds that are aqueous are split into ionsNa aq Cl - aq Ag aq NO 3-.
Masonic Freemasonry architecture-columns set Ionic - Doric - Corinthian 2200 Quantity In Stock. 67cm 264 in Weight.
 Classical Orders Doric Ionic Corinthian Column Cap By Yesteeyear Cafepress
Classical Orders Doric Ionic Corinthian Column Cap By Yesteeyear Cafepress
Instead it was considered as a late Roman form of the Corinthian order.

Ionic doric and corinthian columns. The structural integrity of these columns is commendable in providing support to the buildings. Doric Ionic and Corinthian. The three major classical orders are Doric Ionic and Corinthian.
They have a capital the top or crown made of a circle topped by a square. 550gr 11 lb Ionic Order Column Height. The shaft the tall part of the column is plain and has 20 sides.
Ionic Doric and Corinthian columns are the architectural orders that originated in the classic era of ancient Greeks. These three styles have several distinctive elements. Corinthian columns Corinthian columns were named after Corinth its a city in Greece developed in Athens and rarely used in Greece but common in Rome Italy.
Doric columns were stouter than those of the Ionic or Corinthian orders. Ionic Corinthian and Doric made up of the capital shaft and base. All these three orders had three separate parts of the base shaft and the capital.
The Doric order was one of the three orders of ancient Greek and later Roman architecture. However the most recognizable distinctions are found in the columns. 65cm 256 in Weight.
Corinthian Ionic and Doric in Ancient Greece Doric Columns. Until the Renaissance it was not ranked as a separate order. 26 cm 1025 in Width.
Ionic columns are taller and thinner with a decorative foot and scroll-shaped volutes on the capital. Doric is the simplest and oldest of the three Greek architectural orders while the Ionic is the second order which was later developed. The height of columns are calculated in terms of a ratio between the diameter of the shaft at its base and the height of the column.
The other two canonical orders were the Ionic and the Corinthian. Their smooth round capitals are simple and plain compared to the other two Greek orders. These orders were later adopted by the Romans.
Each column consists of a base a central axis and a capital. 25 cm 984 in Width. A Doric column has a very plain straightforward design much more simple than the later Ionic and Corinthian column styles.
The ancient Roman military architect Vitruvius c. The Doric is most easily recognized by the simple circular capitals at the top of columns. However the Doric Ancient Greek Columns had no base.
The Composite order is a mixed order combining the volutes of the Ionic with the leaves of the Corinthian order. 70-15 BC wrote that Ionic design was an appropriate combination of the severity of the Doric and the delicacy of the Corinthian. A Doric column can be described as seven diameters high an Ionic column as eight diameters high and a Corinthian column nine diameters high although the actual ratios used vary considerably in both ancient and revived examples but keeping to the trend of.
The capital of the Doric order is simple. The column of the Composite order is ten diameters high. The Greek architecture is delineated by three main styles.
For this reason the Doric column is sometimes associated with strength and masculinity. As shown in Figure 4 the Corinthian is similar to the Ionic order in its base column and entablature but its capital is far more ornate carved with two tiers of curly acanthus leaves. Ionic columns are characterized by the capital which is formed with two opposed volutes spiral scrolls and stands on a base of stacked disks.
The Greek Doric column was fluted or smooth-surfaced and had no base dr. The three orders are Doric Ionic and Corinthian columns and even though these columns have progressed through time they still share many similarities. It has thin columns that have grooves running vertically up the sides.
Doric columns were stouter than those of the Ionic or Corinthian orders. The Doric the Ionic and the Corinthian were unique styles invented by the ancient Greeks. Of the three columns found in Greece Doric columns are the simplest.
Postage 400 to Turkey Standard Shipping Get Additional Rates. 730gr 161 lb Corinthian Order Column Height. 07 cm 276 in Weight.
895gr 197 lb Material. The Parthenon in Athens built in the mid fifth century BC is the iconic example of the Greek Doric. The corinthian order is one of the three main classical orders styles of ancient Greek and Roman architecture.
These architectural orders differ from one another in their structure and outlook. The Corinthian is the most decorative of the three orders. Originating in the western Doric region of Greece it is the earliest and in its essence the simplest of the orders though still with complex details in the entablature above.
The shaft is fluted and more slender. The most complex order is the Corinthian order which is tall and thin and features a decorative foot volutes and acanthus leaves on the capital. The Ionic column is identified by the scroll at the top as seen on the columns of the The Temple of.
A Doric column is also thicker and heavier than an Ionic or Corinthian column. Select Country ZipPost Code Quantity Get Shipping Rates. The Ancient Greeks introduced the three main orders of columns that we still have today.
Ionic columns are said to be a more feminine response to the earlier Doric order. Doric Order Column Height. 27 cm 1063 in Width.
The Ionic columns create a grand and Classical entrance to the dome. Doric columns are huge and stocky while the Ionic columns are more slender and taller. The first order introduced was the Doric column.
The oldest known Corinthian column stands inside the 5th-century temple of Apollo Epicurius at Bassae. The columns support the entablature which includes the frieze. The other two are the Doric order and Ionic order.
Doric columns dont have a base while Ionic columns have a base.