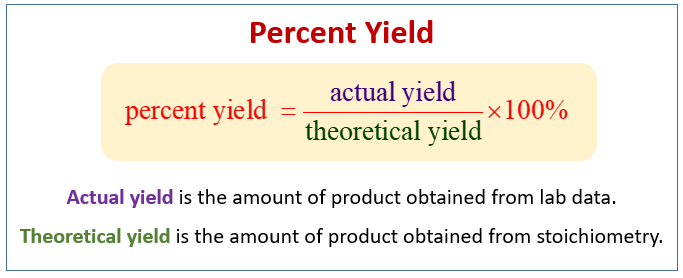
Yield actual yield theoretical yield x 100. The theoretical yield is the amount predicted by a stiochiometric calculation based on the number of moles of all reactants present.
 Stoichiometry And Percent Yield Examples Solutions Worksheets Videos Games Activities
Stoichiometry And Percent Yield Examples Solutions Worksheets Videos Games Activities
What is the percent yield of a reaction in which 455 g of tungstenVI oxide WO3 reacts with excess hydrogen gas to produce metallic tungsten and 797 ml of water d100 gml Please explain.

What is the percent yield of a reaction. What is the percent yield. Yield 385 g Fe 4546214541 grams Fe x 100 8468 or 847. So the yield of this reaction is 80.
Your actual yield is 385 g Fe. In chemical reaction engineering yield conversion. The percentage yield shows how much product is obtained compared to the maximum possible mass.
Check all that apply. Calculating percentage yield The percentage yield is calculated using this equation. Can you have a percent yield over 100.
So what if the sodium hydroxide is not in excess at least we dont know if it is. In chemistry yield also referred to as reaction yield is a measure of the quantity of moles of a product formed in relation to the reactant consumed obtained in a chemical reaction usually expressed as a percentage. Before calculating the yield of a reaction necessary when preparing the laboratory notebook it is crucial to know the stoichiometry of a reaction adjusting the chemical reaction so that there are the same numbers and types of atoms.
The percent yield is the ratio of the actual yield to the theoretical yield expressed as a percentage. Percent yield of the reaction is the percent ratio of actual yield to the theoretical yield. Percentage yield frac actual yield theoretical yield times 100 The percentage yield can vary.
Also a yield of 50 is considered adequate. The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. And 80 yield would be very good.
It is calculated to be the experimental yield divided by theoretical yield multiplied by 100. TextPercent Yield fractextActual YieldtextTheoretical Yield times 100 Percent yield is very important in the manufacture of products. Excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide.
So plug it in. If the percent yield is 100 the actual yield will be equal to the theoretical yield. A reaction yield of 90 percent of the theoretical possible would be considered excellent.
The percent yield of a reaction is the actual yield divided by the theoretical yield x100 or yield actual yieldtheoretical yield x100. Part 1 Finding the Limiting Reactant. Outline the steps needed to determine the percent yield of a reaction that produces 125 g of CCl 2 F 2 from 329 g of CCl 4.
Percent yield actual yieldtheoretical yield x 100. The atom economy of a reaction gives the percentage of atoms in reactants that form a desired product. Normally in order to measure the efficiency of a chemical reaction in organic synthesis the relative or percentage yield is calculated.
For the reaction NH4NO3 s N2O g 2H2O l you decompose 60 g of NH4NO3 and get 11 g of N2O. To express the efficiency of a reaction you can calculate the percent yield using this formula. The other product of this reaction is HCl.
Usually percent yields are understandably less than 100 as we discussed above. Theoretical yield is the amount of product obtained from the stoichiometric or balanced equation using the limiting reactant to determine product. Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis processes.
The reaction yield absolute yield of a chemical reaction is the amount of pure and dry product yielded in a reaction. When you perform this reaction what substances could remain at the end of the reaction. Percent yield actual yieldtheoretical yield x 100 actual yield 6 molecules of NH3 theoretical yield 8 molecules of NH3 the remaining N2 could give two more NH3 so yield 68 x 100.
What is the percent yield of the reaction. Its going to be 04 moles over 05 moles times 100 and we have 80. The equation for percent yield is.
The percent yield of this reaction is going to be the actual yield divided by the theoretical yield multiplied by 100. Since less than what was calculated was actually produced it means that the reactions percent yield must be smaller than 100. Actual yield is the amount of product obtained from a chemical reaction.
Yield actual yieldtheoretical yield x 100. Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long lifetime in the atmosphere. However after you do the experiment you discover that only 650 g of water were produced.
A percent yield of 90 means the reaction was 90 efficient and 10 of the materials were wasted they failed to react or their products were not captured.