Nonmetals are elements showing less or no metallic properties. Non-Metals are present on the right side of the periodic table.
 Matter And Energy Metals Nonmetals And Metalloids Texas Gateway
Matter And Energy Metals Nonmetals And Metalloids Texas Gateway
Metalloids-Metalloids are the group of certain elements which has both the.

Metals metalloids and nonmetals. They are solids under standard conditions and can easily form alloys with Metals. Metals are the elements which exhibit the highest degree of metallic behavior is known as metals on the contrary Non-metals are such elements which do not possess any metallic behavior and Metalloids are those elements that possess some of the properties like metal while some like non-metal. And have acidic oxides.
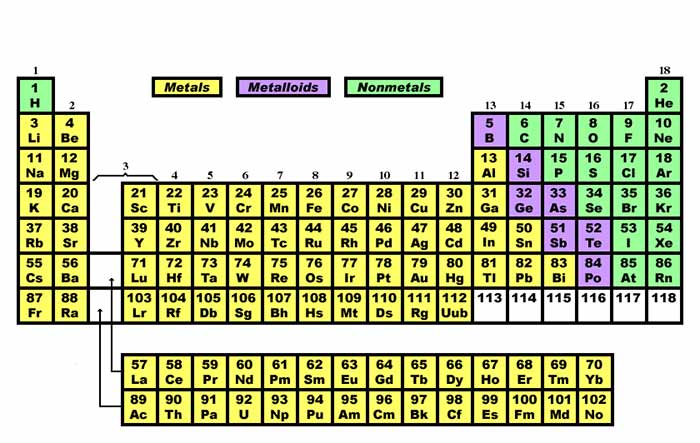
Metals Nonmetals and Metalloids. Metalloids are located to the right of metals and to the left of nonmetals in the periodic table. The metalloids are intermediate in their properties.
Difference Between Metals Nonmetals and Metalloids Definition. Metals Metalloids and Nonmetals Essay Compare and contrast the three different types of elements using their physical properties and explain how you are able to determine if an element is a metal nonmetal or metalloid. These elements are called metalloids.
1 This group of elements is made up of all different states of matter solid liquid and gas a Nonmetals b Metals c Metalloids 2 These elements always have a shiny luster a Nonmetals b Metals c Metalloids 3 These elements are located in a stair step pattern on the periodic table a Nonmetals b Metals c Metalloids 4 Elements in this group are good conductors of heat AND. Difference Between Metals and Nonmetals. Are brittle when solid.
Metalloids have properties intermediate between the metals and nonmetals. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Position in the Periodic Table.
Metalloids tend to be economically important because of their unique conductivity properties they only partially conduct electricity which make them valuable in the semiconductor and computer chip industry. Your essay must have an introduction 1 paragraph a body 3 paragraphs and a conclusion 1 paragraph. An Introduction to General Organic and Biological Chemistry 12th Identify each of the following elements as a metal a nonmetal or a metalloid.
They have lustre shine They do not have lustre shine They are malleable. They can form alloys with other metals. They cannot be broken into thin sheets.
Non-metals do not have properties present in metals whereas metalloids are elements that have intermediate properties of both metals and non-metals. Metals are elements having the highest degree of metallic behavior. Metals Nonmetals and Metalloids Add to my workbooks 0 Download file pdf.
Metals Metalloids and Nonmetals metals nonmetals and metalloids comparison ID. Where is between metals and metalliods. Nonmetals are usually poor conductors of heat and electricity and are not malleable or ductile.
Most of the metals are solids at room temperature with a characteristic silvery shine except for mercury which is a liquid. They can be broken into thin sheets They are non malleable. The main difference between metals non-metals and metalloids are that metals are elements that are hard malleable fusible shiny ductile and good conductors.
To understand the basic properties separating Metals from Nonmentals and Metalloids An element is the simplest form of matter that cannot be split into simpler substances or built from simpler substances by any ordinary chemical or physical method. Also include the location of the elements on the periodic table. Typical nonmetals have a dull coloured or colourless appearance.
In their physical properties they are more like the nonmetals but under certain circumstances several. Examples of non-metals are carbon nitrogen etc. Metalloids are metallic-looking brittle solids that are either semiconductors or exist in semiconducting forms and have amphoteric or weakly acidic oxides.
The metalloids or semimetals have properties that are somewhat of a cross between metals and nonmetals. Trends based on Groups Formal Charges 99 Practice Problems. Where at the metalloids located on the Periodic Table.
Metalloids are all solid at room temperature. SOLIDS LIQUIDS or GASSES. Many of the elemental nonmetals are gases at room temperature while others are liquids and others are solids.
Are poor conductors of heat and electricity. Metalloids are elements having a low degree of metallic behavior. Metalloids Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals.
Can a metalloid have properties of metals and nonmetals. Metalloids are useful in the semiconductor industry. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
Nonmetals are elements showing less or no metallic properties. Also include the location of the elements on the periodic table.
 How Can We Identify Elements As Metal Or Non Metals Quora
How Can We Identify Elements As Metal Or Non Metals Quora
The main difference between metals non-metals and metalloids are that metals are elements that are hard malleable fusible shiny ductile and good conductors.

How to identify metals nonmetals and metalloids. And have acidic oxides. This worksheet can be used to test students by having them identify elements as metals nonmetals or metalloids. A few elements have properties that are either anomalous given their category or otherwise extraordinary.
However metalloids have a shiny and dull appearance. Metalloids are useful in the semiconductor industry. Non-metals do not have properties present in metals whereas metalloids are elements that have intermediate properties of both metals and non-metals.
Properties of Metals Nonmetals and Metalloids are discussed and you see where to find them on the periodic table. Position in the Periodic Table. For example using a.
We compiled videos featuring Metals Nonmetals and Metalloids. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. Metals have a shiny appearance non-metals have a dull appearance.
The line begins at boron B and extends down to polonium Po. Metals can be found on the left side of the periodic table while nonmetals are found on the right side. Nov 13 2020 BIOLOGY MODULE Animal Cells.
How to Identify Metals Nonmetals and Metalloinds. One useful way is by metals nonmetals and metalloids. Metals Nonmetals and Metalloids on the Periodic Table - YouTube A description and practice of finding metals nonmetals and metalloids on the Periodic TableIn general metals are found on the.
Elements to the left of the line are considered metals. It is usually easy to tell metals and non-metals apart but some tests are more reliable than others. Using the periodic table you can classify the elements in many ways.
Are poor conductors of heat and electricity. Metals are located in s p d and f blocks in the periodic table though non-metals is located in s and p blocks and metalloids are located in p block of the periodic table. They can form alloys with other metals.
Your essay must have an introduction 1 paragraph a body 3 paragraphs and a conclusion 1 paragraph. Using the electron configuration and a periodic table answer the following questions. Typical nonmetals have a dull coloured or colourless appearance.
Notice that metals and non-metals have opposite properties to each other. Difference Between Metals Nonmetals and Metalloids Definition. Metals are elements having the highest degree of metallic behavior.
Elements to the far right of the periodic table are nonmetals. Somewhere between metals and nonmetals lie metalloids or semiconductors Let us identify the. Are brittle when solid.
Metals Nonmetals and Metalloids. It also has a section to list the physical characteristics of each type of element. Metalloids are elements having a low degree of metallic behavior.
Metalloids are metallic-looking brittle solids that are either semiconductors or exist in semiconducting forms and have amphoteric or weakly acidic oxides. Metals and nonmetal answer key. Metals and nonmetal answer key displaying top 8 worksheets found for this concept.
Metalloids have properties intermediate between the metals and nonmetals. Metals Nonmetals and Metalloids Overview. The periodic table is organized in families and periods.
Most or some elements in each category share a range of other properties. Physical Chemical Properties of Metals and Nonmetals. This worksheet can be used to test students by having them identify elementsas metals nonmetals or metalloids.
Metalloids are all solid at room temperature. Metals Metalloids and Nonmetals Essay Compare and contrast the three different types of elements using their physical properties and explain how you are able to determine if an element is a metal nonmetal or metalloid. It also has a section to list the physical characteristics of each type of element.
In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84. Most elements can be considered metals.
 The Parts Of The Periodic Table
The Parts Of The Periodic Table
The line begins at boron B and extends down to polonium Po.
/Periodic-Table-Metals-56a12db33df78cf772682c44.png)
Periodic table showing metals. Atomic structure and the periodic table Elements in group 1 and group 2 are metals. Elements to the left of the line are considered metals. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right.
It organizes elements according to an idealized orbital filling instead of valence. At ordinary temperatures and pressures hydrogen behaves as a nonmetal. Except for Germanium Ge and Antimony Sb all the elements to the left of that line can be classified as metals.
Atoms to the left of the. Metals reside on the left side of the table while non-metals reside on the right. Most periodic tables print a thick black line to show the division between metals and nonmetals.
Major alternative structures Left-step periodic table Janet 1928 Charles Janets left-step periodic table is the most widely used alternative to the traditional depiction of the periodic system. Alkaline Earth Metals. Alloys such as brass and bronze also are metals.
A section of the periodic table showing metals and non-metals The main groups are numbered from 1 to 7 going from left to right and the last group on the right is group 0. The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties. Periodic Table or Periodic Chart of Elements showing alkali metals.
For example the elements Sc to Zn are shown as a 3d block implying orbital occupancy Ar 4s 2 3d x. They are grouped together in the middle to the left-hand side of the periodic table. And atomic number 52 Antinomy Sb.
The archetypal transition metals and the physically and chemically weak post-transition metals. The highlighted elements are considered the metal elements. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc.
The exception is hydrogen H the first element on the periodic table. Elements to the far right of the periodic table are nonmetals. All of the alkaline earth metal atoms have a 2 oxidation state.
Atomic number 32 Germanium Ge. Periodic Table of the Elements - Gases Links to Tables showing Periodic Element Groups. Periodic Table of the Elements GROUPS Alkali Metals Alkaline Earth Metals Blocks Gases stp Halogens LanthanidesActinides Liquids stp Main Group Metalloids Metals Noble Gases Non-Metals Solids stp Transition Metals.
From left to right in the periodic table these categories include the highly reactive alkali metals. Metals comprise the large majority of the elements and can be subdivided into several different categories. Metals Nonmetals and Metalloids on the Periodic Table - YouTube A description and practice of finding metals nonmetals and metalloids on the Periodic TableIn general metals are found on the.
There are only two exceptions ie two elements in that sequence between number 5 and number 84 that are not metals. The alkaline earth metals are found in group IIA of the periodic table which is the second column of elements. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals.
The section in the. Like the alkali metals these elements are found in compounds rather than pure form. Atoms of group 1 elements have one.
Metals are on the left of the periodic table and non-metals are on the right. Most of the elements on the periodic table are metals including gold silver platinum mercury uranium aluminum sodium and calcium. If you look at the Periodic table you will find that the metal elements are located between atomic number 5 Boron B all the way to atomic number 84 Polonium Po.
The less reactive alkaline earth metals lanthanides and radioactive actinides. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. Alkali Metals Alkaline Earth Metals Blocks Gases Halogens LanthanidesActinides Liquids stp Main Group Metalloids Metals Noble Gases Non-Metals Solids stp Transition Metals Periodic.
Location of Metals on the Periodic Table Metals are located on the left side and the middle of the periodic table. The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. This line is often referred to as the staircase because of its shape.
Non-Metal materials are non-crystalline in nature. It has a density of 270 gcm3.
How Are Metals Classified Quora
Understand how to use electrical conductivity and the acid-base character of oxides to classify elements as Metals or Non-metals.

How to classify metals. Identifying Common Types of Metals Aluminum. The basic metals make up the element to the right of the transition metals. When it comes into.
Color As A Clue in Metal Identification Methods. Metals vs Non-Metals samabrhms11 2019-05-30T1439350100 Specification Point 120. The transition elements groups IB to VIIIB are also considered metals.
Here are the codes for some of the more common metals. I Elements which contain 1 to 3 electrons in their outermost shell are metals. Basic Classification of Engineering Materials Metals.
The most common way of classifying them is by their iron content. This includes the alkali metals alkaline earth metals transition metals lanthanides and actinides. A UNS number cant totally replace other codes however since it doesnt provide complete information about the metals properties.
This guidance will help you to correctly classify iron and steel products ranging from base metals waste and scrap and other primary forms through treated and semi-manufactured items to specific. Metals and non-metals can also be distinguished by some chemical properties. This metal is grey and shiny but it doesnt sparkle.
Metals form oxides that are basic but. The most common chemical property is the type of oxide that the element forms. One of the way to classify metals is on the basis of their structure.
Metals are located on the left side and the middle of the periodic table. Metals and non-metals have different properties. Elements containing 4 to 7 electrons in their valence shell are non-metals.
Metals have a rather interesting structure which comprises of metal ions and a sea of free electronslet me help you visualise this structure. Non-metals are elements that tend to gain electrons and form covalent bonds. Different chemical models have different features and limitations.
Metals that do not have any iron content are non-ferrous metals. Properties of metals can be explained in terms of metallic structure and bonding. The iron imparts magnetic properties to the material and also makes them prone to corrosion.
Except for Germanium Ge and Antimony Sb all the elements to the left of that line can be classified as metals. However you could differentiate them by trying. On the periodic table metals are separated from nonmetals by a zig-zag line stepping through carbon phosphorus selenium iodine and radon.
It can differentiate precious metals magnesium aluminum brass and copper. Metals are elements present in the periodic table. Are good conductors of heat and electricity.
Group IA and Group IIA the alkali metals are the most active metals. When a metal contains iron it is known as a ferrous metal. And have at least one basic oxideMetalloids are metallic-looking brittle solids that are either.
These codes begin with UNS followed by a letter and 5-digit number. Published 21 December 2018 Last updated 31 December 2020 see all updates. Elements are analyzed in a ground powder form.
Hence A and C are metals whereas B D and E are non-metals. Most elements are metals. These exists in amorphic or mesomorphic forms.
A strong clue in metal identification is color. These elements and those to the right of them are nonmetals. A00001 to A99999 Aluminum and aluminum alloys C00001 to C99999 Copper and copper alloys.
Selected Jan 25 2018 by Vikash Kumar. Ii Type of bonds. Find a commodity code to classify your goods and look up duty rates reliefs and quotas.
In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84. XRD can be used hand in hand with XRF as XRD takes the testing one step further to give added context. Form alloys with other metals.
The process identifies the crystalline phases present and compares them to a database of archived phases. Its often confused with gold because they have the same color. X-Ray Diffraction XRD is used to identify chemical composition information of metals.
The chemical elements can be broadly divided into metals metalloids and nonmetals according to their shared physical and chemical propertiesAll metals have a shiny appearance at least when freshly polished. Metals are elements that tend to lose electrons and form metallic bonds which allow them to conduct electricity. If there are signs of oxidation remove it via scraping to reveal the color of the unoxidized surface.
Metals are polycrystalline bodies which are having number of differentially oriented fine crystals.
The center of the periodic table contains the transition metals plus the two rows below the body of the table lanthanides and actinides are special transition metals. They are separated by a diagonal band of semimetals.
Most elements are metals.
Where on the periodic table are metals located. Astatine atomic number 85 shows characteristics of nonmetals halogens as well as metalloids. This line is often referred to as the staircase because of its shape. If you look at the Periodic table you will find that the metal elements are located between atomic number 5 Boron B all the way to atomic number 84 Polonium Po.
Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals. The elements on the left of the periodic table are metals. Except for Germanium Ge and Antimony Sb all the elements to the left of that line can be classified as metals.
In other words metalloids semimetals are located on the right side of the post transition metals and on the left side of nonmetals see above image. Beryllium Be Magnesium Mg Calcium Ca Strontium Sr Barium Ba and Radium Ra. Alkaline earth metals are located just next to the alkali metals.
Inner transition metals are located in the two rows at the bottom of the periodic table. The metals share several common properties including. And atomic number 52 Antinomy Sb.
Alkaline earth metals includes. The orange color on the Periodic table represents metalloids. Metalloids have properties of both metals and nonmetals.
The p-block elements include all of the nonmetals except for hydrogen and helium the semimetals and the post-transition metals. Most periodic tables print a thick black line to show the division between metals and nonmetals. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides.
The elements to left of the line are metals. Atoms to the left of the. Atomic number 32 Germanium Ge.
They form a separating boundary between the metals and nonmetals. All these nonmetals are located on the upper right corner of the Periodic table Hydrogen is located on the left top corner In the above image the nonmetals are represented in yellow color. Groups 3-12 Transition Metals or Transition Elements The d and f block metals have 2 valence electrons.
Seeing chemical elements arranged in the modern periodic table is as familiar as seeing a map of the world but it was not always so obvious. Metals are found to the left of the stair step line. Lanthanides Ce Lu having atomic number from 58 71 and.
These two rows at the bottom of the Periodic table are called. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right. According to some chemists the d-block elements are known as transition elements.
Most elements can be considered metals. From the above image you can easily see where are Transition Metals located on the Periodic Table. The line begins at boron B and extends down to polonium Po.
Metalloids are located between the metals and nonmetals. In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84. Metals are lustrous good conductors of electricity and readily shaped they are ductile and malleable whereas solid nonmetals are generally brittle and poor electrical conductors.
The noble gases are almost completely inert. Also many periodic tables have a stair-step line on the table identifying the element groups. The three broad categories of elements are metals nonmetals and metalloids.
P-block elements include the last six element groups of the periodic table excluding helium. The creator of the periodic table Dmitri Mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. Metals are located on the left of the periodic table and nonmetals are located on the upper right.
From above image you can easily find where Inner Transition Metals are located on Periodic Table. They are grouped together in the middle to the left-hand side of the periodic table. The elements on the stair step line are metalloids The elements to the right of the line are non metals.
Where are Transition Metals located on the Periodic Table. There are only two exceptions ie two elements in that sequence between number 5 and number 84 that are not metals. Nonmetals are located on the righthand side of the periodic table.
From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases. Metals have a relatively low electron negativity. The transition metals are located in the middle of the Periodic table from group 3 to group 11.
There are 18 nonmetals on the Periodic table. The metalloids separate the metals and nonmetals on a periodic table. The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine.
This above image shows you where are alkaline earth metals found on the Periodic table.
