Any help to gain insights into the derivation of this formula would be helpful. KE ½ 17kg 5ms² 2125 J.
 Kinetic Energy Equation In Joules Kg M S 2 タイトルロゴ
Kinetic Energy Equation In Joules Kg M S 2 タイトルロゴ
Kinetic energy is typically measured in units of Joules and 1 Joule is equal to 1 kilogram-meters squared per second squared.
Formula for kinetic energy chemistry. To figure the total kinetic energy you multiply the average kinetic energy by the number of molecules you have which is nNA where n is the number of moles. The formula for the energy of motion is. The formula for calculating kinetic energy KE is KE 05 x mv 2.
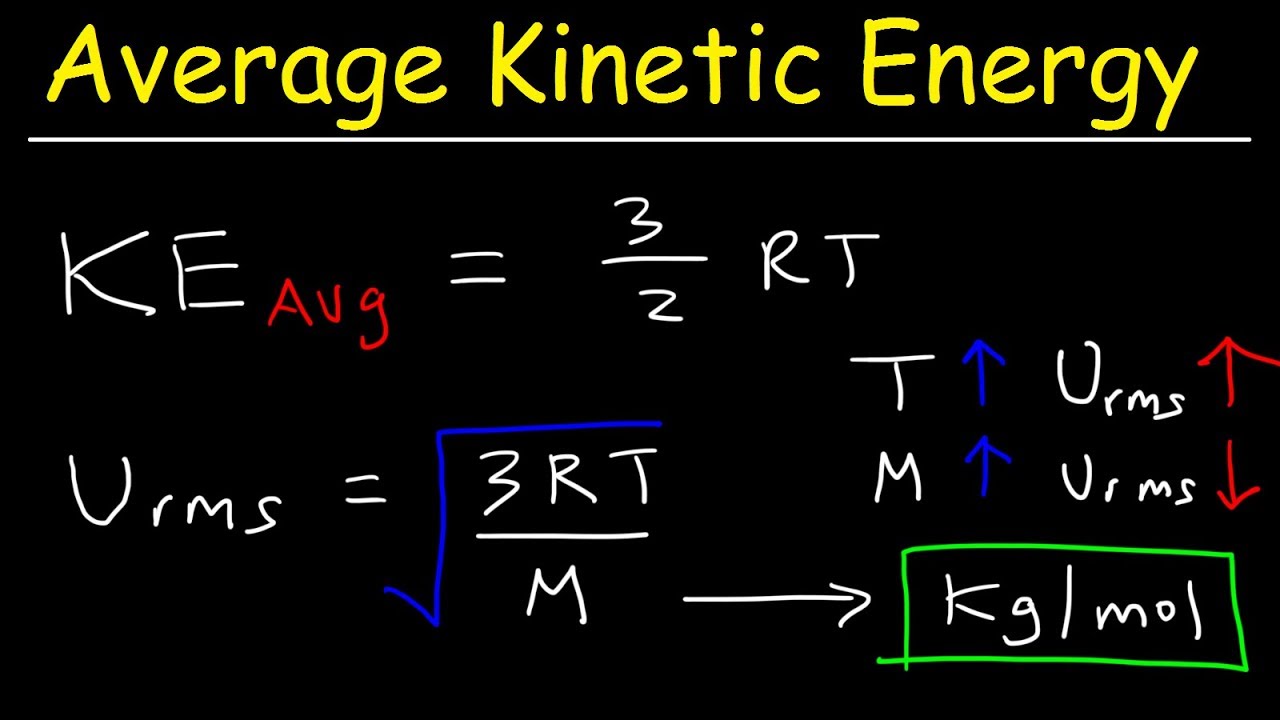
The kinetic energy of the translational motion of an ideal gas depends on its temperature. Kinetic Energy Solved Examples. But if I equate them then f comes out to be 3.
It is equivalent to 1 kg m 2 s 2. KE05times mtimes v2 where KE is kinetic energy in joules m is mass in kilograms and v is velocity in meters per second. Where the four-velocity of a particle is.
The formula for kinetic energy of an object is K 12mv2. NAk equals R the universal gas constant so this equation becomes the following. The formula for kinetic energy is KE.
Underneath are questions on Kinetic energy which aids one to understand where they can use these questions. 12 mv2 where m stands for mass and v stands for velocity. Potential energy is energy that comes from position and a force.
Translational kinetic energy of a body is equal to one-half the product of its mass m and the square of its velocity v or 12mv2. Where m is the mass of the molecule. Interscale kinetic energy transfer in chemically reacting compressible isotropic turbulence - Volume 912.
Here m stands for mass the measure of how much matter is in an object and v stands for the velocity of the object or the rate at which the object changes its position. The kinetic energy is measured in Joules J and the temperature is measured in Kelvin K. This formula is valid only for low to relatively high speeds.
It contains plenty of. Big things moving fast have the most energy the most ability to shove other things or knock them over etc. Energy is usually divided into two types by chemists.
This chemistry video tutorial explains how to calculate the average kinetic energy of a gas and the root mean square velocity as well. So the sphere has a total kinetic energy of 2125 J. Your answer should always be stated in joules J which is the standard unit of measurement for kinetic energy.
U α d x α d τ displaystyle u alpha frac dx alpha dtau and. τ displaystyle tau is the proper time of the particle there is also an expression for the kinetic energy of the particle in general relativity. I have read 2 formulas for kinetic energy of 1 mol of gas - 32 RT and 12fRT.
The formula for the kinetic energy of a gas defines the average kinetic energy per molecule. Are we supposed to memorize and know how to solve for average kinetic energy. For a simple monoatomic gas like helium or neon the only motion that the atoms can do is to move from one place to another in a straight line until they bump into something else such as another atom or molecule86 This kind of motion is called translational motion and is directly linked to the kinetic energy of the atom or molecule through the relationship KE 12 m vbar2 32 kT where vbar is the average velocity of all of the molecules in the population87 m is the mass k is a.
The equation is not on the equation sheet You do not have the required permissions to view the files attached to this post. Solution for The average translational kinetic energy for a molecule Erans is given by the following equation. K average kinetic energy per molecule of gas J.
Kinetic energy formula is used to compute the mass velocity or kinetic energy of the body if any of the two numerics are given. For extremely high-speed particles it yields values that are too small. Kinetic energy is the energy that occurs in an object in motion.
If the particle has momentum. I read from a book that average kinetic energy is equal to 3 k T 2 where k is Boltzmanns constant and T is the kelvin temperature. I dont know how the formula was derived.
Using our equation we can determine the kinetic energy of the sphere. This definition should make sense. As an example to illustrate kinetic energy lets say that a 17 kg sphere is moving in a straight line with a velocity of 5 ms.
The equation for kinetic energy is 1 K E 1 2 m v 2 where KE is kinetic energy m is mass and v is velocity. But f is different of mono di polyatomic gases. The Kinetic energy is articulated in Kgm 2 s 2.
By signing up youll get thousands of step-by-step.