Iodide is a halide anion and a monoatomic iodine. The halogens are the elements in Group 7 of the periodic table.
Their ions are called halide ions eg.
Iodine cation or anion. It is assigned a radius of around 206 picometers. By contrast hydrogen fluorine and iodine only need to gain one electron so they form an anion with a charge of negative one. This is the iodide ion i there are compounds though where it actually forms a cation.
Some salts of the anion have been isolated including thalliumI triiodide Tl I 3 and ammonium triiodide NH 4 I 3. Tests for anions Testing for halide ions The halogens are the elements in group 7 of the Periodic Table and include chlorine bromine and iodine. Cation vs anion periodic table.
It has a role as a human metabolite. Solutions containing iodide ions are typically colourless in colourTo test for the presence of iodide ions in solution you may use the leadII nitrate tes. Learn vocabulary terms and more with flashcards games and other study tools.
For comparison the lighter halides are considerably smaller. Some common elements that form anions are hydrogen fluorine iodine and oxygen. Most other metals form cations eg.
Iodide is one of the largest monatomic anions. Sodium iodide is the salt of a very strong acid and thus its salt is essentially neutral. It is formed by combining aqueous solutions of iodide salts and iodine.
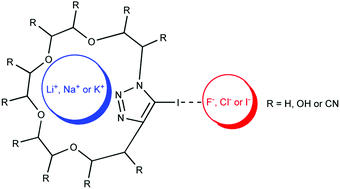
While molecular iodine is competent as a catalyst in its own right the triazolium cation triples the reaction rate and reaches turnover frequencies up to 30 h 1 presumably through beneficial interactions of the electron-poor azolium π system and I 2 which facilitate the electron transfer from the substrate to iodine and concomitant formation of I. Please visit the Iodine element page for information specific to the chemical element of the periodic table. It is a conjugate base of a hydrogen iodide.
Bromide 196 pm chloride 181 pm and fluoride 133 pm. Compounds with iodine in formal oxidation state 1 are called iodides. Learn vocabulary terms and more with flashcards games and other study tools.
It has a role as a human metabolite. This anion one of the polyhalogen ions is composed of three iodine atoms. This is the iodide ion I- There are compounds though where it actually forms a cation.
Their ions are called halide ions eg chloride Cl-. The absence of a precipitate with fluoride ions doesnt prove anything unless you already know that you must have a halogen present and are simply trying to find out which one. Most iodide salts are soluble in water but often less so than the related chlorides and bromides.
On the other hand because HF is a relatively weak acid so sodium fluoride a salt of HF is weakly basic. Iodine 1 is a monoatomic monocation and a monoatomic iodine. Oxygen needs to gain two electrons so it forms an anion with a charge of negative two.
Aqueous solutions of iodide dissolve iodine better than pure water due to the formation of complex ions. Iodide is a halide anion and a monoatomic iodine. Triiodide is observed to be a red colour in solution.
Iron silver nickel whilst most other nonmetals typically form. This is the iodide ion i there are compounds though where it actually forms a cation. Halogens always form anions alkali metals and alkaline earth metals always form cations.
Chlorine bromine and iodine are halogens. Therefore we expect Iodine to have a. The element Iodine will exist as an anion with a charge of -1.
1 structures expand this. In part because of its size iodide forms relatively weak bonds with most elements. It is reported that iodine forms compounds like C H X 3 C O O X 3 I I C l O X 4 X 3 and I P O X 4 which contains I X and I X 3 and are not bonded covalently as in interhalogen compounds.
Iodine has 7 valence electrons and needs to receive an extra electron to achieve the octet rule. The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound such as sodium chloride. All the absence of a precipitate shows is that you havent got chloride bromide or iodide ions present.
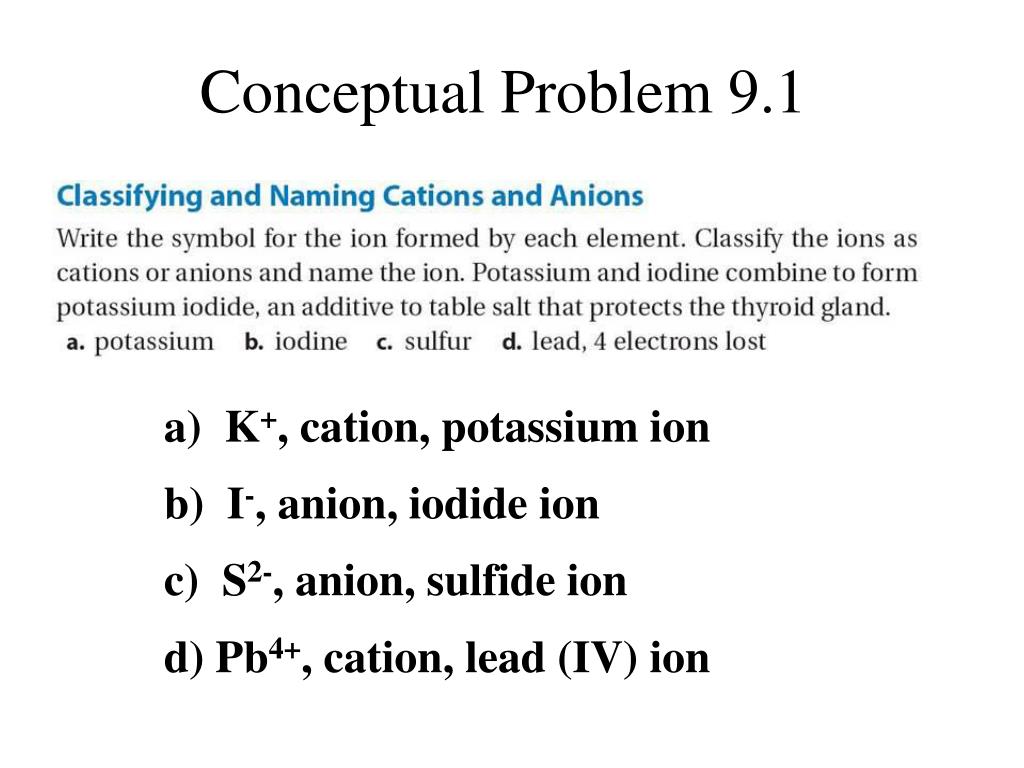
Iodine cation or anion. Iodide has been investigated for the treatment of Goiter Nodular. Cations positively-charged ions and anions negatively-charged ions are formed when a metal loses electrons and a nonmetal gains those electrons.
The chemistry of the test. Generally Halogens form anions of the type X X to achieve stability by attaining noble gas configuration. It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table.
This acceleration is specific for triazolium cations and represents a hybrid anioncation catalytic process as a simple and. Iodine 1 is a monoatomic monocation and a monoatomic iodine. Iodine cation or anion.
Iodine tends to form an anion. It is a conjugate base of a hydrogen iodide. Halide ions in solutions are detected.
Fluoride anion is a stronger base because its the salt of a weaker acid.