Nonmetals are elements showing less or no metallic properties. Non-Metals are present on the right side of the periodic table.
 Matter And Energy Metals Nonmetals And Metalloids Texas Gateway
Matter And Energy Metals Nonmetals And Metalloids Texas Gateway
Metalloids-Metalloids are the group of certain elements which has both the.

Metals metalloids and nonmetals. They are solids under standard conditions and can easily form alloys with Metals. Metals are the elements which exhibit the highest degree of metallic behavior is known as metals on the contrary Non-metals are such elements which do not possess any metallic behavior and Metalloids are those elements that possess some of the properties like metal while some like non-metal. And have acidic oxides.
Metals Nonmetals and Metalloids. Metalloids are located to the right of metals and to the left of nonmetals in the periodic table. The metalloids are intermediate in their properties.
Difference Between Metals Nonmetals and Metalloids Definition. Metals Metalloids and Nonmetals Essay Compare and contrast the three different types of elements using their physical properties and explain how you are able to determine if an element is a metal nonmetal or metalloid. These elements are called metalloids.
1 This group of elements is made up of all different states of matter solid liquid and gas a Nonmetals b Metals c Metalloids 2 These elements always have a shiny luster a Nonmetals b Metals c Metalloids 3 These elements are located in a stair step pattern on the periodic table a Nonmetals b Metals c Metalloids 4 Elements in this group are good conductors of heat AND. Difference Between Metals and Nonmetals. Are brittle when solid.
Metalloids have properties intermediate between the metals and nonmetals. A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. Position in the Periodic Table.
Metalloids tend to be economically important because of their unique conductivity properties they only partially conduct electricity which make them valuable in the semiconductor and computer chip industry. Your essay must have an introduction 1 paragraph a body 3 paragraphs and a conclusion 1 paragraph. An Introduction to General Organic and Biological Chemistry 12th Identify each of the following elements as a metal a nonmetal or a metalloid.
They have lustre shine They do not have lustre shine They are malleable. They can form alloys with other metals. They cannot be broken into thin sheets.
Non-metals do not have properties present in metals whereas metalloids are elements that have intermediate properties of both metals and non-metals. Metals are elements having the highest degree of metallic behavior. Metals Nonmetals and Metalloids Add to my workbooks 0 Download file pdf.
Metals Metalloids and Nonmetals metals nonmetals and metalloids comparison ID. Where is between metals and metalliods. Nonmetals are usually poor conductors of heat and electricity and are not malleable or ductile.
Most of the metals are solids at room temperature with a characteristic silvery shine except for mercury which is a liquid. They can be broken into thin sheets They are non malleable. The main difference between metals non-metals and metalloids are that metals are elements that are hard malleable fusible shiny ductile and good conductors.
To understand the basic properties separating Metals from Nonmentals and Metalloids An element is the simplest form of matter that cannot be split into simpler substances or built from simpler substances by any ordinary chemical or physical method. Also include the location of the elements on the periodic table. Typical nonmetals have a dull coloured or colourless appearance.
In their physical properties they are more like the nonmetals but under certain circumstances several. Examples of non-metals are carbon nitrogen etc. Metalloids are metallic-looking brittle solids that are either semiconductors or exist in semiconducting forms and have amphoteric or weakly acidic oxides.
The metalloids or semimetals have properties that are somewhat of a cross between metals and nonmetals. Trends based on Groups Formal Charges 99 Practice Problems. Where at the metalloids located on the Periodic Table.
Metalloids are all solid at room temperature. SOLIDS LIQUIDS or GASSES. Many of the elemental nonmetals are gases at room temperature while others are liquids and others are solids.
Are poor conductors of heat and electricity. Metalloids are elements having a low degree of metallic behavior. Metalloids Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals.
Can a metalloid have properties of metals and nonmetals. Metalloids are useful in the semiconductor industry. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
Where at the metalloids located on the Periodic Table. Periodic table separation of metals from nonmetals_Properties of metals metalloids and nonmetals WikipediaProperties of metals metalloids and nonmetals The periodic table showing.
 Metalloids Chemistry For Non Majors
Metalloids Chemistry For Non Majors
Elements in the metalloids class fall in between the metals and nonmetals in their properties.

Periodic table showing metals nonmetals and metalloids. SOLIDS LIQUIDS or GASSES What makes up most of the periodic table What is the classification of these characteristics. Metals are placed on the left side of the periodic table Non-metals are placed on the right side of the periodic table and Metalloids are placed in the middle of the periodic table. 1 This group of elements is made up of all different states of matter solid liquid and gas a Nonmetals b Metals c Metalloids 2 These elements always have a shiny luster a Nonmetals b Metals c Metalloids 3 These elements are located in a stair step pattern on the periodic table a Nonmetals b Metals c Metalloids 4 Elements in this group are good conductors of heat AND.
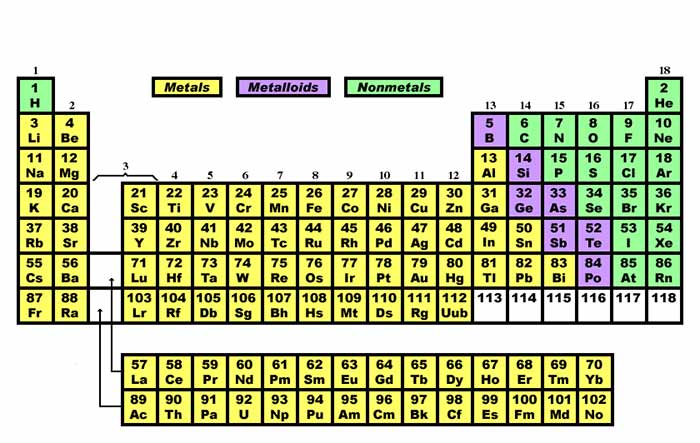
Metals Nonmetals and Metalloids Using the periodic table you can classify the elements in many ways. There are 118 elements known to us out of which 92 are naturally occurring while the rest have been prepared artificially. Elements are further classified into metals non-metals and metalloids based on their properties which are correlated with their placement in the periodic table.
The metalloids separate the metals and nonmetals on a periodic table. Block in the. The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine.
Boron B silicon Si germanium Ge arsenic As antimony Sb tellurium Te polonium Po and astatine At are the elements found along the step like line between metals and non-metals of the periodic table. Where are the nonmetals located on the Periodic Table. Sporadically recognised elements show that the metalloid net is sometimes cast very widely.
The noble gases are almost completely inert. The periodic table is organized in families and periods. 9182015 24543 PM.
Metals in most of the left and centre. Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals. Properties of Metals Nonmetals Metalloids Created Date.
Although they do not appear in the list of metalloid lists isolated. Nonmetals class generally cannot. From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases.
3 DIFFERENT TYPES OF ELEMENTS. The orange color on the Periodic table represents metalloids. Atomic structure and the periodic table Elements in group 1 and group 2 are metals.
Also many periodic tables have a stair-step line on the table identifying the element groups. Metalloids have properties of both metals and non-metals. An example of a metalloid is arsenic As.
Periodic Table of the Elements Mg meta loids. Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals. Metalloids in a narrow.
Metals Nonmetals and Metalloids on the Periodic Table - YouTube A description and practice of finding metals nonmetals and metalloids on the Periodic TableIn general metals are found on the. They form a separating boundary between the metals and nonmetals. The line begins at boron B and extends down to polonium Po.
In the periodic table you can see a zig-zag line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84. In other words metalloids semimetals are located on the right side of the post transition metals and on the left side of nonmetals see above image. Metals are elements having the highest degree of metallic behavior.
Summarize how the periodic table is organized in terms of metals nonmetals and metalloids. Why is this information useful. Arsenic is the element in opening photo B In the periodic table above elements are color coded to show their class.
Where is the right side. Metals are found in the left side of the periodic table. Atoms of group 1 elements have one.
Where are metals located on the periodic table Most metals are. Percentages are median appearance frequencies in the list of metalloid lists. Difference Between Metals Nonmetals and Metalloids Definition.
One useful way is by metals nonmetals and metalloids. Metals are located in s p d and f blocks in the periodic table though non-metals is located in s and p blocks and metalloids are located in p block of the periodic table. Also we can say that metalloids are present in the diagonal region of the p block on Periodic table.
METALS NONMETALS METALLOIDS Classifying elements on the Periodic Table. Metals are on the left of the periodic table and non-metals are on the right. Position in the Periodic Table.
Periodic table extract showing groups 12 and 1218 and a dividing line between metals and nonmetals. Metals non-metals and metalloids.
Elements to the left of the line are considered metals. They are on the right side of the periodic table.
The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine.

Where are nonmetals on the periodic table. Atomic structure and the periodic table Elements in group 1 and group 2 are metals. Periodic table model science software exploring the periodic table ppt online exploring the periodic table ppt online periodic table of elements help by james butler chem 11 see his Comment 1 One Reply to Where Are The Metals Nonmetals And Lanthanides Located On Periodic Table. The nonmetals are brittle not malleable or ductile poor conductors of both heat and electricity and tend to gain electrons in chemical reactions.
A description and practice of finding metals nonmetals and metalloids on the Periodic TableIn general metals are found on the left-hand side of the period. Which type of elements make up most of the periodic table. Some nonmetals C black P S and Se are brittle solids at room temperature although each of these also have malleable pliable or ductile allotropes.
Also Know what is the most chemically reactive non metal. Nonmetals on the Periodic Table The nonmetals are located on the upper right side of the periodic table. Elements with no metallic properties ME.
Element with physical and chemical properties of metals and nonmetals 20. What is an ion. Shiny element ductile malleable good conductor of electricity and thermal energy NM.
How many nonmetals are there on the Periodic table. Metals are lustrous good conductors of electricity and readily shaped they are ductile and malleable whereas solid nonmetals are generally brittle and poor electrical conductors. The most reactive nonmetals reside in the upper right portion of the periodic table.
They are separated by a diagonal band of semimetals. Metals are on the left of the periodic table and non-metals are on the right. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals.
These elements are shown in the following figure. And they are not ductile you cannot make them into thin wires or malleable they can not be made into thin sheets. Since the noble gases are a special group because of their lack of reactivity the element fluorine is the most reactive nonmetal.
Non-metals can be easily located on the Periodic Table because they are to the right of the line that looks like a stepping ladder. Non-metals are characterized by having the exact opposite properties of metals. So non-metals are brittle instead of solid.
Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals. The only chemical element that is not found in this region of the table is Hydrogen which is located in the upper left corner together with Alkaline Metals but since it behaves in most circumstances as a Non-Metal it is classified as such. The only exception to this is atomic number 1 Hydrogen H which has a different location on the table.
The nonmetals are mainly in the upper right of the Periodic Table. These elements are often referred to as other nonmetals as the halogens and noble gases are also nonmetals. The nonmetals list which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc.
At room temperature they are sometimes gases or brittle solids. These elements have similar chemical properties that differ from the elements considered metals. Helium as an s-block element would normally be placed next to hydrogen and above beryllium.
Nonmetals and metalloids in the periodic table. There are 18 nonmetals on the Periodic table. It is not found in nature as a free element.
Nonmetals have properties opposite those of the metals. In the periodic table in this chapter they are in green. They are located to the right of the metalloids and to the left of the halogens.
Elements to the far right of the periodic table are nonmetals. ADVERTISEMENT When we study the elements it is important to know which elements are metals and which ones are not. The nonmetals are a group of elements in the periodic table.
The highlighted elements are the nonmetal elements. Some nonmetals are liquids. All these nonmetals are located on the upper right corner of the Periodic table Hydrogen is located on the left top corner In the above image the nonmetals are represented in yellow color.
The exception is hydrogen H the first element on the periodic table. Apart from hydrogen nonmetals are located in the p-block. From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases.
The nonmetal elements occupy the upper right-hand corner of the periodic table. Describe properties of metals nonmetals and metalloids. However since it is a noble gas it is instead placed above neon in the p-block.
Metals are located on the left of the periodic table and nonmetals are located on the upper right. The nonmetals in the periodic table. Nonmetals include the nonmetal group the halogens and the noble gases.
Atoms of group 1 elements have one. Nonmetals are poor conductors of heat and electricity.