The reactant that is all used up is called the limiting reactant - it sets a limit on how much product can form. Calculate the mole ratio from the.
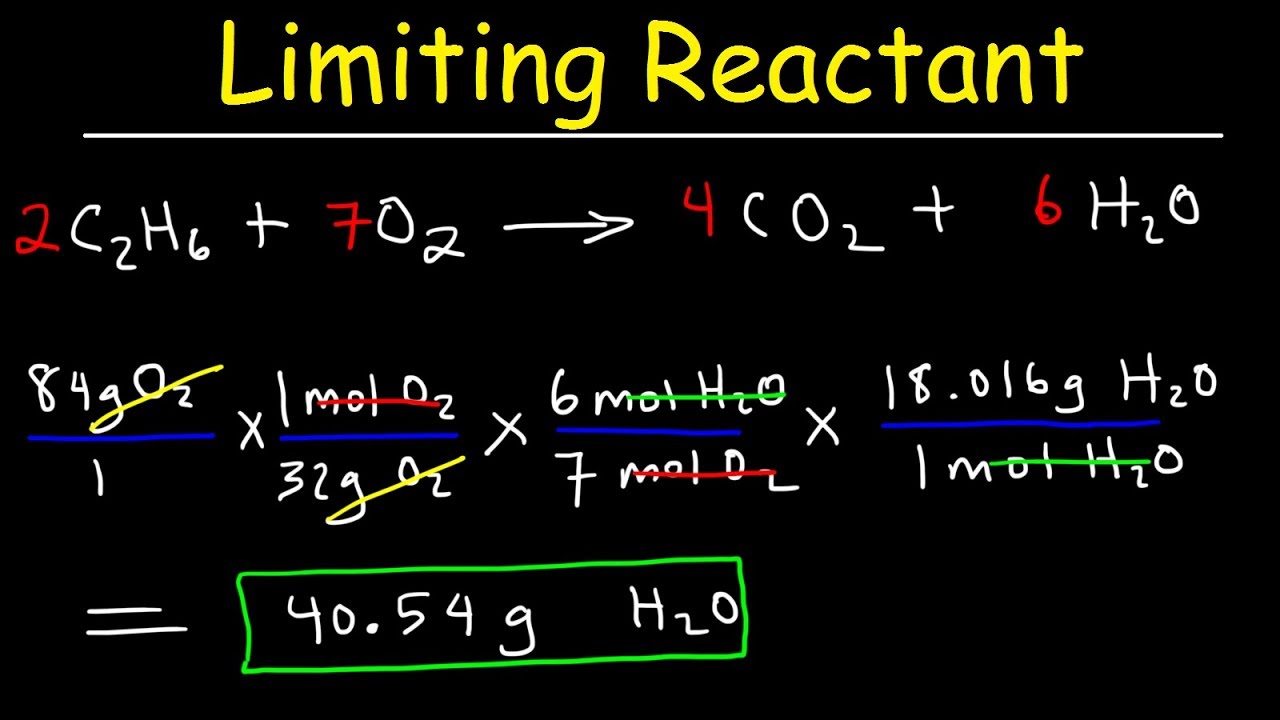
 How To Find Limiting Reactant Quick Easy Examples Practice Problems Practice Questions Youtube
How To Find Limiting Reactant Quick Easy Examples Practice Problems Practice Questions Youtube
Limiting reactant Use the atomic masses of Ag and S to determine the number of moles of each present.
How to determine limiting reactant. A value less than the ratio means the top reactant is the limiting reactant. In this case if we react 100 of Na2CO3 which is 005000 mol how much of HCl is required. The reactant that yields the smallest mass of product is the limiting reactant.
Dont waste good thought. The reactant that forms the least amount of product will be the limiting reactant. Compare this result to the actual number of moles of sulfur present.
The reactant which is in a lesser amount than is required by stoichiometry is the. Limiting reactant in a reaction is found by calculating the amount of product produced by each reactant. The reactant that is left over is described as being in excess.
Calculate the yield of each reactant. Enter any known value for each reactant the limiting reagent will be highlighted. The first is to compare the actual mole ratio of the reactants to the mole ratio of the balanced chemical equation.
In this example you are beginning with 9 times as much oxygen as glucose when measured by number of moles. To determine which reactant is the limiting reactant first determine how much product would be formed by each reactant if all the reactant was consumed. If we divide our moles of H 2 into moles of N 2 our value will tell us which reactant will come up short.
The reactant that is all used up is called the limiting reactant the reactant that is left over is described as being in excess The mass of product formed in a reaction depends upon the mass of the. Identify the excess reagent. If youre seeing this message it means were having trouble loading external resources on our website.
The other method is to calculate the gram masses of the product resulting from each reactant. Based on the calculation we need 01000 mol of HCl to react with 100 of Na2CO3 but we only have 008000 mol of HCl. This chemistry tutorial covers how to find the limiting reagent when given amounts of different reactants and how to calculate the theoretical yield using th.
Compare the two ratios you calculated to identify the limiting reactant. There are two methods used to find the limiting reactant. When there are only two reactants write the balanced chemical equation and check the amount of reactant B required to.
Any value greater than the above ratio means the top reactant is in excess to the lower number. Next to determine the limiting reagent we calculate the amount of reactant B required to completely react with all of reactant A. The following points should be considered while attempting to identify the limiting reagent.
So heres the solution. The mass of product. Therefore you have more oxygen than required.
Determine the limiting reagent if 100 g of ammonia and 100 g of oxygen are present at the beginning of the reaction. Learn how to identify the limiting reactant in a chemical reaction and use this information to calculate the theoretical and percent yields for the reaction. Determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation.
Determine the balanced chemical equation for the chemical reaction. Before doing anything else you must have a balanced reaction equation. Convert all given information into moles most likely through the use of molar mass as a conversion factor.
The reactant that produces the least amount of product is the limiting reactant. Then use the balanced equation to calculate the number of moles of sulfur that would be needed to react with the number of moles of silver present. The formula tells you that your ideal ratio is 6 times as much oxygen as glucose.
How do you find the limiting reactant with ML. How To Calculate Limiting Reagents. Calculate the number of moles of each reactant by multiplying the volume of each solution by its molarity.
Identify the limiting reactant and determine the mass of CO2 that can be produced from the reaction of 250g of C3H8 with 750g of O2. The balanced chemical equation is already.