Nonelectrolyte definition is - a substance that does not readily ionize when dissolved or melted and is a poor conductor of electricity. Solutions of nonelectrolytes do not conduct electricity.
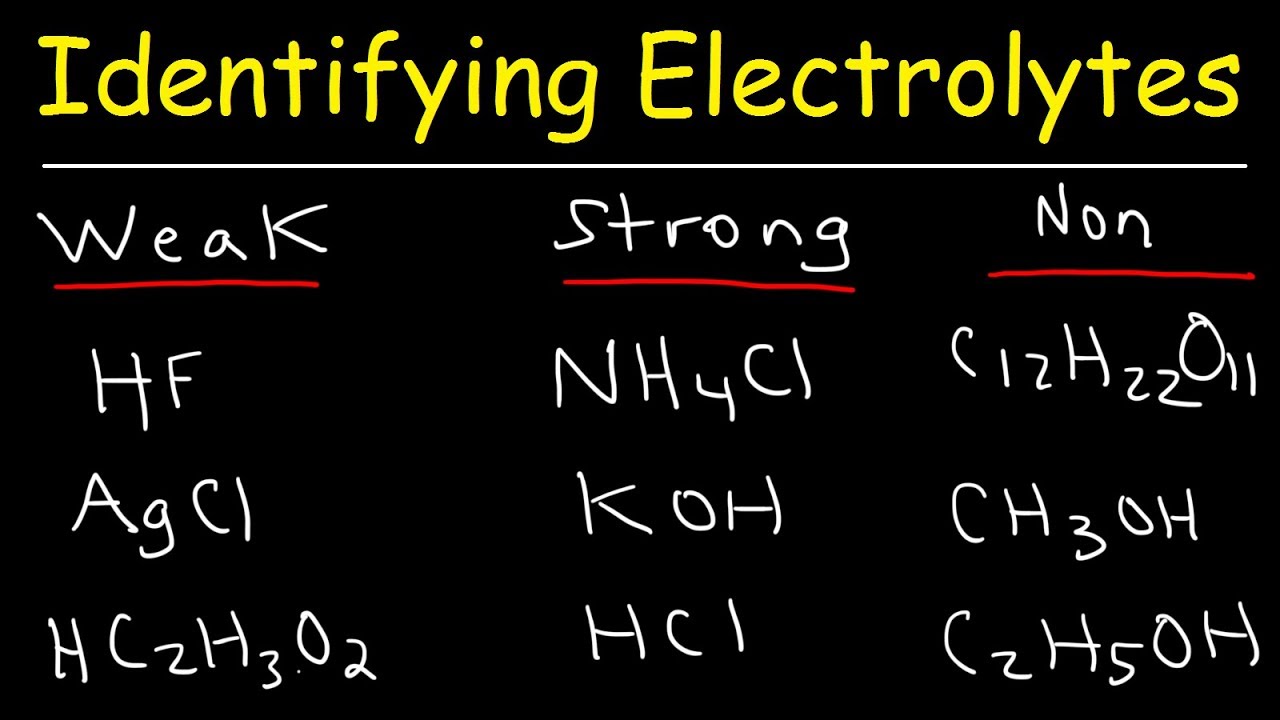 Identifying Strong Electrolytes Weak Electrolytes And Nonelectrolytes Chemistry Examples Youtube
Identifying Strong Electrolytes Weak Electrolytes And Nonelectrolytes Chemistry Examples Youtube
Updated July 03 2019.

What is a nonelectrolyte. Nonelectrolytes can be defined as substances that do not have any distinct ionic form to exist in when they are dissolved in an aqueous solution. The matter that settles to the bottom of a liquid. Published by Houghton Mifflin Company.
Search the Dictionary for More Terms. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. Unlike electrolytes nonelectrolytes do not conduct electricity when in a solution.
Many molecular compounds such as sugar or ethanol are nonelectrolytes. When these compounds dissolve in water they do not produce ions. Nonelectrolyte synonyms nonelectrolyte pronunciation nonelectrolyte translation English dictionary definition of nonelectrolyte.
The difference between the two classes gave rise to the view that there are two types of chemical bond. Most carbon compounds are nonelectrolytes. A substance that dissolves in water to produce a liquid that electricity does not go through 2.
2 strong acids and 3 strong base. The bulb remains unlit. Return to top of page.
Water is considered a weak electrolyte by some sources because it partly dissociates into H and OH ions but a nonelectrolyte by other sources because only a very small amount of water dissociates into ions. A nonelectrolyte does not dissociate at all in solution and therefore does not produce any ions. Strong electrolytes are substances that are completely ionized when they are dissolved in water several classes of strong electrolytes.
A nonelectrolyte is a term used in chemistry to denote a substance that does not break up or dissociate into ions when placed in solution. Nonelectrolytes usually consists of molecules which are covalently bonded and may or may not dissolve in water. A common example of a nonelectrolyte is glucose or C 6 H 12 O 6.
Typically nonelectrolytes are primarily held together by covalent rather than ionic bonds. N a substance that does not easily ionize in a solution and thus conducts electricity poorly Collins English Dictionary Complete and Unabridged 12th. Sugar C12H22O11 is a good example of a nonelectrolyte.
As a result solutions containing nonelectrolytes will not conduct electricity. When these compounds dissolve in water they do not produce ions. Many molecular compounds such as sugar or ethanol are nonelectrolytes.
Medical Dictionary for the Health Professions and Nursing Farlex 2012. Electrolyte substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Nonelectrolytes are compounds that do not ionize at all in solution.
Such substances are usually poor conductors of electricity due to the fact that they do not readily dissociate into ions in their melt state or in their dissolved state. Nonelectrolytes are typically polar covalent substances that do dissolve in water as molecules instead of ions. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution.
Nonelectrolytes tend to be poor electrical conductors and dont readily dissociate into ions when melted or dissolved. C A sucrose solution which is a nonelectrolyte contains no ions and does not conduct a current. Nonĕ-lektrō-līt A substance with molecules that do not in solution dissociate to ions and therefore do not carry an electric current.
A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. The American Heritage Stedmans Medical Dictionary Copyright 2002 2001 1995 by Houghton Mifflin Company. If a substance doesnt ionize in water at all its a nonelectrolyte.
Glucose sugar readily dissolves in water but because it does not dissociate into ions in solution it is considered a nonelectrolyte. Ionic and covalent compounds Members of the other class nonelectrolytes dissolve to yield solutions that do not conduct electricity. What is a Nonelectrolyte.
The state of being isolated kept apart or withdrawn into solitude. TAKE THE QUIZ TO FIND OUT. Weak acids and weak bases are weak electrolytes.
Also what is the difference between an electrolyte and a Nonelectrolyte. A substance that dissolves in water to produce a liquid that electricity does not go through 2. Click to see full answer.
Fats sugars and alcohols are largely nonelectrolytes.