How to Draw a Lewis Structure Step 1. A step-by-step explanation of how to draw the N2 Lewis Dot Structure Nitrogen Gas - Diatomic NitrogenFor the N2 structure use the periodic table to find t.
 A Simple Guide For Learning How To Draw Lewis Dot Structures Teaching Chemistry Chemistry Classroom Chemistry Education
A Simple Guide For Learning How To Draw Lewis Dot Structures Teaching Chemistry Chemistry Classroom Chemistry Education
Write the atomic symbol for each atom.
How to draw lewis dot structures. The example below should shed some light on this. Bond Pair Electrons 2 x No. How to Draw Lewis Dot Structures Method 1of 3.
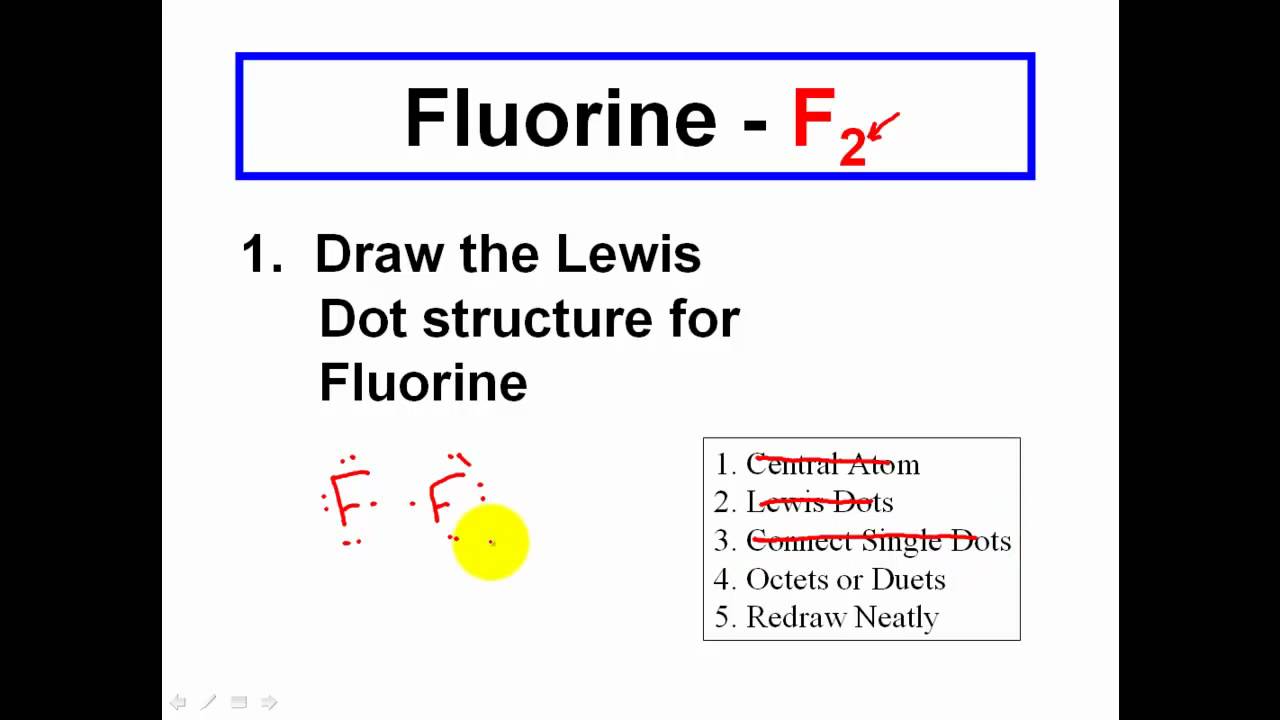
A video tutorial for how to draw Lewis Structures in five steps. Lewis structures are drawn to help one understand or predict how many types of bonds can be formed around a given atom. How to Draw a Lewis Dot Structure Step 1.
Determine the Number. Write the 2 atomic symbols. CO 2 Total 16 Step 2.
If there are multiple elements that have more dots than the rest then just pick one at random among the higher dotted elements. Ketzbook demonstrates how to draw Lewis diagrams for elements and simple molecules using an easy to follow step-by-step explanation with several examplesLew. It helps in predicting the quantity and types of bond formed around an atom ion or molecule.
Place least electronegative element in center and draw single bonds from the central atom to other atoms. Examples of Lewis Structures. Purpose of drawing a Lewis Dot Structure.
A step-by-step explanation of how to draw the Al2O3 Lewis Dot StructureFor Al2O3 we have an ionic compound and we need to take that into account when we dra. Calculations to Draw Lewis Structures. COUNT THE TOTAL VALENCE ELECTRONS.
In Lewis dot structure only valence electrons are used for making of the structure. Drawing Diatomic Covalent Structures. Determine which atom is your central atom.
A Draw the lewis structure by repeating steps 1-4. A Lewis structure is a pictorial diagram showing how electrons get distributed around an atom. A step-by-step explanation of how to draw the SeO3 2- Lewis Dot StructureFor the SeO3 2- structure use the periodic table to find the total number of valenc.
An atom is considered happy when its outer. A step-by-step explanation of how to write the Lewis Dot Structure for NH4 Ammonium IonFor the NH4 Lewis structure calculate the total number of valenc. It will also work with more complex molecules and ions if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures.
Find the Total Number of Valence Electrons. In this step add up the total number of valence electrons from all. Determine the total number of valence electrons to be depicted in the Lewis diagram.
Creating Lewis Structures for Larger Covalent Molecules. Stages to articulate the electron dot formula are stated beneath. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols.
Determine how many. The drawing is started by determining types of covalent bonds that are formed after combining atoms. The video covers the basic Lewis structures youll see in an introductory chemistry class.
A Lewis Electron Dot Formula comprises one dot for every valence electron and an elements symbol. Valence electrons are the electrons present in the outermost shell of the electronic configuration of an atom. When drawing Lewis Dot Structures for ionic compounds you need to follow a different set of rules than with Lewis Structures for covalentmolecular compounds.
Refer to the first picture b find the element s that has more dots than the rest. A step-by-step explanation of how to draw the NH2I Lewis Dot StructureFor the NH2I structure use the periodic table to find the total number of valence elec. How to Draw Electron Dot Structures.
Lewis structures back up the concepts of general chemistry such as chemical bonding resonance electron pair repulsion theory etc. Find the Number of Electrons Needed to Make the Atoms Happy. Lone pair electrons can be on lone pair of double and triple bond electrons.
Q Valence electrons of all the atoms any negative charge any positive charge Where Q Total Electrons. Lewis structure helps in deriving the geometry of the molecules. How to Draw Lewis Structures.
Lone Pair Electrons Q Bond Pair Electrons. The following is a general procedure for drawing Lewis structures.